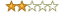<< 1 >>
Rating: 
Summary: Useful for a newcomer to the clinical trial environment
Review: As a clinical trials computer programmer, I'm not the primary target audience for this book. However, as an experienced programming consultant who was new to clinical studies in the pharmaceutical industry, I found it gave me a useful overview of the typical deliverables that my efforts supported. Acronyms that are frequently used in the clinical environment, such as IB (Investigator's Brochure) and ISS (Integrated Safety Study) are given some definition and examples are shown. I found this information valuable in enabling me to feel comfortable with these concepts when they were discussed in meetings with statisticians and project managers.
Rating: 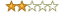
Summary: Not a practical guide, more a manifesto !
Review: Dr. Bonk is a seasoned professional who has worked at all levels of his craft, including the highest. This book is entitled "a practical guide for pharmaceutical research", and specifically concentrates on the output of documents from the pharmaceutical industry (regulatory documents and manuscripts, as well as, to a lesser extent, audio-visual preparations and computer-generated products). I bought the book because it was the only one that specifically addresses the needs of those of us working in the pharmaceutical industry. This book is a manifesto, or one (alternative) philosophy, for the role of the medical writer in a pharmaceutical company. As such, its greatest achievement will be to attract people with talent into an occupation that is sorely in need of more and better writers. If this book stimulates under-graduates or graduates to explore this profession, then it will have made a significant, contribution to pharmaceutical research.
However, this book falls far short of being a practical guide. It is not, nor does it claim to be, a style guide; this is not a deficiency in itself. But to be a practical guide, there is much more that it needs to consider: how do medical writers multi-task, and manage their inevitably numerous assignments all at varying degrees of completion ? What should one look for in word processing software, and is this the same for everybody ? How can one enhance one's interactions with journal editors ? What are the relevant professional guilds and associations, and what are their courses and credentials like ? These are a few of the practical things, outside questions of style, that medical writers need to work inside the pharmaceutical industry.
The document templates provided (and lauded in the book's advertising) are poor. The regulatory documents should have followed closely the ICH or FDA guidelines (neither is new). A reprinted, stellar example of a published paper, with some illuminating commentary, and even some succes!sive stages of drafting, would have been an asset.
The initial sections of this book attempt to teach about the drug development process. Unless this can be done more thoroughly (and preferably by someone who has actually developed drugs), this description is inadequate to equip a medical writer in a pharmaceutical company. The descriptions are out of date and garbled in places. There are medical writers who indeed significantly contribute to drug development in the way that Dr. Bonk envisages, but they know a lot more than this about how new drugs are discovered and approved.
Lesser criticisms include the production of this book. At a little over 100 loosely worded pages it is not inexpensive. But its printing is cheap, and the figures were especially poorly printed in my copy. The figures, too, were often redundant; for example, the "pyramids" of the components of a medical journal manuscript were especially irrelevant.
In summary, this book is unique in giving a sound philosophy for the medical writer in a pharmaceutical company. As a practical guide (its stated claim) it is a real failure. This reviewer now understands why its plaudits were written by Professors of English in liberal arts schools, and not by experienced pharmaceutical industry personnel.
A.W.Fox, MD, PhD, FFPM
Rating: 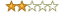
Summary: Not a practical guide, more a manifesto !
Review: Dr. Bonk is a seasoned professional who has worked at all levels of his craft, including the highest. This book is entitled "a practical guide for pharmaceutical research", and specifically concentrates on the output of documents from the pharmaceutical industry (regulatory documents and manuscripts, as well as, to a lesser extent, audio-visual preparations and computer-generated products). I bought the book because it was the only one that specifically addresses the needs of those of us working in the pharmaceutical industry.
This book is a manifesto, or one (alternative) philosophy, for the role of the medical writer in a pharmaceutical company. As such, its greatest achievement will be to attract people with talent into an occupation that is sorely in need of more and better writers. If this book stimulates under-graduates or graduates to explore this profession, then it will have made a significant, contribution to pharmaceutical research. However, this book falls far short of being a practical guide. It is not, nor does it claim to be, a style guide; this is not a deficiency in itself. But to be a practical guide, there is much more that it needs to consider: how do medical writers multi-task, and manage their inevitably numerous assignments all at varying degrees of completion ? What should one look for in word processing software, and is this the same for everybody ? How can one enhance one's interactions with journal editors ? What are the relevant professional guilds and associations, and what are their courses and credentials like ? These are a few of the practical things, outside questions of style, that medical writers need to work inside the pharmaceutical industry. The document templates provided (and lauded in the book's advertising) are poor. The regulatory documents should have followed closely the ICH or FDA guidelines (neither is new). A reprinted, stellar example of a published paper, with some illuminating commentary, and even some succes!sive stages of drafting, would have been an asset. The initial sections of this book attempt to teach about the drug development process. Unless this can be done more thoroughly (and preferably by someone who has actually developed drugs), this description is inadequate to equip a medical writer in a pharmaceutical company. The descriptions are out of date and garbled in places. There are medical writers who indeed significantly contribute to drug development in the way that Dr. Bonk envisages, but they know a lot more than this about how new drugs are discovered and approved. Lesser criticisms include the production of this book. At a little over 100 loosely worded pages it is not inexpensive. But its printing is cheap, and the figures were especially poorly printed in my copy. The figures, too, were often redundant; for example, the "pyramids" of the components of a medical journal manuscript were especially irrelevant. In summary, this book is unique in giving a sound philosophy for the medical writer in a pharmaceutical company. As a practical guide (its stated claim) it is a real failure. This reviewer now understands why its plaudits were written by Professors of English in liberal arts schools, and not by experienced pharmaceutical industry personnel. A.W.Fox, MD, PhD, FFPM
<< 1 >>
|