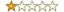<< 1 >>
Rating: 
Summary: Great organic chemistry book
Review: Dr. Atkins, I use Dr. loosely, should stop writing books. He assume the reader knows to much. Also, Dr. Atkins should stay in his own realm of chemistry, Physical chemistry and not try to intrude into other areas. Avoid this book like the plague. Atkins writes only to write them and get a fat check. The content is sub-par.
Rating: 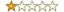
Summary: Never buy an Atkins Book
Review: Dr. Atkins, I use Dr. loosely, should stop writing books. He assume the reader knows to much. Also, Dr. Atkins should stay in his own realm of chemistry, Physical chemistry and not try to intrude into other areas. Avoid this book like the plague. Atkins writes only to write them and get a fat check. The content is sub-par.
Rating: 
Summary: Great organic chemistry book
Review: P.W. Atkins's Molecules is probably one of the best general interest books written about organic chemistry. The 2nd edition is much expanded from the 1st, with more molecules and a much slicker presentation. Though the book is written for non-chemists, it's also useful for people in the field, who perhaps know the chemistry, but not the applications (if you walk into any chemist's office, look at his or her bookshelves, and can find a general interest book, chances are it's this one). Maybe the only thing bad I can say about the 2nd edition is that, unlike the 1st edition, it doesn't include the molecular line structures that organic chemists normally use, opting instead for the more colorful, but less useful, RasMol depictions.
Rating: 
Summary: Explores the molecule nature of life
Review: This is a popular book on organic chemistry, a celebrated one at that, this being the second edition, substantially revised. The first was published in 1987. It is one of those almost legendary books of the publishing history, a technical book on a highly technical subject that somehow managed to reach something close to a large readership.Ironically, the reason is not so much in the drawings of the molecules, but in the text. Peter Atkins covers a wide range of interesting molecules and shows how they are related, and he makes their properties semi-accessible to the general reader. I say, "semi" because, frankly for this chemistry-challenged person, seeing two-dimensional shapes of the molecules helps me to understand them only slightly. I suspect for those more conversant with chemistry, the drawings (new for this edition) will be valuable. To me, the mystery of why a certain shape and elemental composition should result in a nutritious substance whereas something else with only the slightest change should be poisonous is not dispelled. He begins with "Simple substances," oxygen molecules, nitrogen, our air and its pollutants. He ends with the very complex DNA and RNA. Along the way he enlightens us about so many of the chemicals and foods and consumer products we use in our daily lives from soaps and gasoline to fats and oils, to painkillers and street drugs. His style is very readable and he has the welcome knack of being informative about interesting things. Here are some examples: Baking power releases carbon dioxide to leaven baked goods in two separate bursts. "The first burst occurs at room temperature as a result of the action of the moistened tartaric acid...The second...is due to the action of the aluminum salt, and it occurs at high temperature." (p. 24) One of the differences between synthetic and natural vanilla (vanillin) is that the natural is "weakly radioactive," the former having been made from coal tar, "from which the radioactivity has long decayed," while the latter picks up some radioactive carbon-14 atoms captured from the atmosphere during photosynthesis. (p. 154) (Of course natural vanilla is also more expensive.) Lemons originally came from northern India and were introduced into the Mediterranean region about a thousand years ago. (p. 155) "Initially, a young white wine may have a greenish hue from the chlorophyll...molecules that survive fermentation." (p. 176) Window glass allows UV-A rays to pass through but blocks UV-B rays. (p. 180) I had always wondered about this because I had gotten conflicting information from different sources. There's a Glossary and many full color illustrations and photos on glossy paper in addition to the color-coded drawings of the molecules, some of which are very beautiful. There's an Introduction in which Atkins explains the difference between elements and molecules, between atoms and compounds, and differentiates between the bonds between atoms and the forces that hold molecules together.
Rating: 
Summary: Explores the molecule nature of life
Review: This is a popular book on organic chemistry, a celebrated one at that, this being the second edition, substantially revised. The first was published in 1987. It is one of those almost legendary books of the publishing history, a technical book on a highly technical subject that somehow managed to reach something close to a large readership. Ironically, the reason is not so much in the drawings of the molecules, but in the text. Peter Atkins covers a wide range of interesting molecules and shows how they are related, and he makes their properties semi-accessible to the general reader. I say, "semi" because, frankly for this chemistry-challenged person, seeing two-dimensional shapes of the molecules helps me to understand them only slightly. I suspect for those more conversant with chemistry, the drawings (new for this edition) will be valuable. To me, the mystery of why a certain shape and elemental composition should result in a nutritious substance whereas something else with only the slightest change should be poisonous is not dispelled. He begins with "Simple substances," oxygen molecules, nitrogen, our air and its pollutants. He ends with the very complex DNA and RNA. Along the way he enlightens us about so many of the chemicals and foods and consumer products we use in our daily lives from soaps and gasoline to fats and oils, to painkillers and street drugs. His style is very readable and he has the welcome knack of being informative about interesting things. Here are some examples: Baking power releases carbon dioxide to leaven baked goods in two separate bursts. "The first burst occurs at room temperature as a result of the action of the moistened tartaric acid...The second...is due to the action of the aluminum salt, and it occurs at high temperature." (p. 24) One of the differences between synthetic and natural vanilla (vanillin) is that the natural is "weakly radioactive," the former having been made from coal tar, "from which the radioactivity has long decayed," while the latter picks up some radioactive carbon-14 atoms captured from the atmosphere during photosynthesis. (p. 154) (Of course natural vanilla is also more expensive.) Lemons originally came from northern India and were introduced into the Mediterranean region about a thousand years ago. (p. 155) "Initially, a young white wine may have a greenish hue from the chlorophyll...molecules that survive fermentation." (p. 176) Window glass allows UV-A rays to pass through but blocks UV-B rays. (p. 180) I had always wondered about this because I had gotten conflicting information from different sources. There's a Glossary and many full color illustrations and photos on glossy paper in addition to the color-coded drawings of the molecules, some of which are very beautiful. There's an Introduction in which Atkins explains the difference between elements and molecules, between atoms and compounds, and differentiates between the bonds between atoms and the forces that hold molecules together.
<< 1 >>
|