Arts & Photography
Audio CDs
Audiocassettes
Biographies & Memoirs
Business & Investing
Children's Books
Christianity
Comics & Graphic Novels
Computers & Internet
Cooking, Food & Wine
Entertainment
Gay & Lesbian
Health, Mind & Body
History
Home & Garden
Horror
Literature & Fiction
Mystery & Thrillers
Nonfiction
Outdoors & Nature
Parenting & Families
Professional & Technical
Reference
Religion & Spirituality
Romance
Science
Science Fiction & Fantasy
Sports
Teens
Travel
Women's Fiction
|
 |
Chemistry Connections: The Chemical Basis of Everyday Phenomena, Second Edition |
List Price: $34.95
Your Price: $34.95 |
 |
|
|
|
| Product Info |
Reviews |
<< 1 >>
Rating: 
Summary: Great teacher resource
Review: Chemistry Connections is an excellent, well-written book that I found extremely easy and fun to read. It is a fascinating book for both the layperson and the scientist, presenting explanations of everyday phenomena that all of us have wondered about at one time or another. Things like household cleaners, paper money, seashells, shampoo, and chili pepper are explained on a simplified chemical basis, and then on a more detailed scientific basis. Chemical structures and some reactions are presented, as well as very current references and websites on each topic. This has got to be the most entertaining chemistry book I have every read!
Rating: 
Summary: Fascinating without forsaking the science
Review: Chemistry Connections is an excellent, well-written book that I found extremely easy and fun to read. It is a fascinating book for both the layperson and the scientist, presenting explanations of everyday phenomena that all of us have wondered about at one time or another. Things like household cleaners, paper money, seashells, shampoo, and chili pepper are explained on a simplified chemical basis, and then on a more detailed scientific basis. Chemical structures and some reactions are presented, as well as very current references and websites on each topic. This has got to be the most entertaining chemistry book I have every read!
Rating: 
Summary: An excellent way to bring chemistry into the everyday
Review: Have you always been somewhat fascinated by chemistry? In college was a chemistry minor, and thought it was very exciting. It explans the many whys and hows behind basic everyday chemistry. Is this book super technical, no. Does it have enough science in it to make it credible, definately.
Some of the topics that are included are:
Why are Opals and Pearls Iridescent?
Why are Ice Cubes Cloudy on the Inside?
What Makes a No-Tears Champoo?
How do Sutures Dissolve?
Why do Lightsticks glow?
Are Flamingos Naturally Pink?
There are almost 100 of these types of questions. This book might make a great reference for a teacher, or a student in a science fair. The book covers some basic chemistry, and does a great job in building up some excitement around a subject that some may think is dull.
I really enjoyed the book because it is broken up into sections: gases, solutions, solids, thermodynamics, and then into the questions that apply to those subjects. The book is a fairly easy read, and thankfully isn't too techincal for those of us who may not be so fresh on some of our chemical understanding.
Rating: 
Summary: Great teacher resource
Review: I found Chemistry Connections very interesting and informative. I am a high school science teacher and I already have a strong background in chemistry. The format presents infomation on each question at multiple levels of sophistication, from introductory information accessible without much knowledge of chemistry to in-depth information on specific molecular structures and interactions. This book would be a great resource for chemistry teachers. My only critisism is that the writing style could be a bit more engaging.
Rating: 
Summary: An absorbing intermediate to advanced level chemical text
Review: Now in an updated second edition, Chemistry Connections: The Chemical Basis Of Everyday Phenomena is an amazing volume with chapters based around answers to questions such as "What causes an egg to crack if it's boiled too rapidly?"; "Why do carbonated drinks go flat as they warm?"; "Why do lightsticks glow?"; "How does a timed-release medicine work?"; and much, much more. The collaborative effort of Kerry K. Karukstis (Professor of Chemistry, Harvey Mudd College) and Gerald R. Van Hecke (Professor of Chemistry, Harvey Mudd College), Chemistry Connections is an absorbing intermediate to advanced level chemical text that makes learning chemistry a lot of fun in some unique but quite effective ways.
Rating: 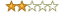
Summary: there are better alternatives
Review: Overall this book and is fairly successful in its aim of explaining a selection of everyday chemical phenomena in terms accssible to most people. There are a couple of points which really let it down though.
First, the presentation of the material and the diagrams could be greatly improved. For example, there is a "3-D" structure of EDTA given. In fact, it just seems to be a 2-D Chemdraw diagram pasted in to Chem3D! It gives no sense of how the dispostion of the oxygen and nitrogen atoms allow EDTA to surround the metal centre thus making it an effective metal sequestering agent.
Second, certain sections go in to extraordinary detail concerning the physical chemistry of certain phenomena. Rather complicated equations and diagrams are presented which don't aid the reader in really understanding conceptualising the processe which are described.
Third, annoyingly, temperatures are presented only in Fahrenheit. Hello!? Did the authors not consider that they may have readers outside of the US.....
(...)
Rating: 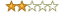
Summary: there are better alternatives
Review: Overall this book and is fairly successful in its aim of explaining a selection of everyday chemical phenomena in terms accssible to most people. There are a couple of points which really let it down though.
First, the presentation of the material and the diagrams could be greatly improved. For example, there is a "3-D" structure of EDTA given. In fact, it just seems to be a 2-D Chemdraw diagram pasted in to Chem3D! It gives no sense of how the dispostion of the oxygen and nitrogen atoms allow EDTA to surround the metal centre thus making it an effective metal sequestering agent.
Second, certain sections go in to extraordinary detail concerning the physical chemistry of certain phenomena. Rather complicated equations and diagrams are presented which don't aid the reader in really understanding conceptualising the processe which are described.
Third, annoyingly, temperatures are presented only in Fahrenheit. Hello!? Did the authors not consider that they may have readers outside of the US.....
(...)
Rating: 
Summary: A good resource for high-school or liberal arts course
Review: There are a lot of books out there explaining the chemistry of everyday things. For sheer readability, I recommend Prof. Joe Schwarcz's series of books, which are readily available on Amazon. The shortcoming of Dr. Joe's books, and most books for the general public, is that they aren't really meant to be a resource for teachers of chemistry. They only include the most basic of explanations that the layman can understand.
Thus, the need for a book like "Chemistry Connections." It contains the same "basic-level" explanation for a layperson, but also has a second explanation for each subject giving details on a more scientifically rigorous scale. Then there's a section of references (often reputable websites, vetted by the authors) for people that want to get even more in depth.
The way the explanations are written, and the choice of topics, makes this a resource best suited to the high-school chemistry classroom, or to a "liberal arts" (chemistry for non-chemists) university course. The explanations tend to be a little thin for a true freshman-level calculus-based chemistry course. That's not to say that a thoughtful professor can't make great use of this book for any level course - the choices of chemical questions are varied and thoughtful, making it a great reference when planning lessons.
The book is not perfect. It does not have the zing or flow of many other books for sheer readability - it's best used as a resource to give ideas for presentations and lesson examples for high school or liberal arts courses. There are some typos - there is a pentavalent organic carbon (horror of horrors!) in one diagram, for example. Finally, some of the detailed explanations tend to wander off-topic, for example, explaining how polymers are synthesised in a section on polymer structure. While interesting, it was not relevant to the particular question at hand, and likely to be confusing for the student.
<< 1 >>
|
|
|
|